IRB Process
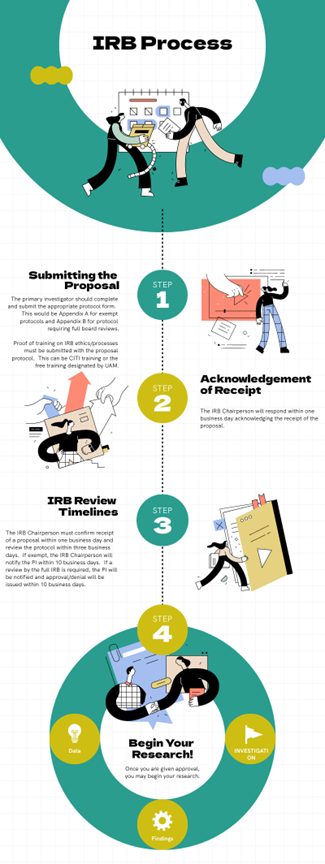
The Institutional Review Board (IRB) process involves several steps to ensure ethical oversight of research involving human subjects. Below is a simplified diagram of the IRB committee’s review process:
- Submission of Research Proposal:
- Researchers submit their research proposal to the IRB.
- The proposal includes details about the study design, informed consent process, and data collection methods.
- Initial Review:
- The IRB reviews the proposal to assess its ethical considerations.
- They evaluate participant protections, risks, and benefits.
- If necessary, the IRB may request modifications or clarifications.
- Exemption Determination (if applicable):
- For studies with minimal risk, the IRB determines if the research qualifies for exemption.
- Exempt studies may not require full board review.
- Expedited Review (if applicable):
- Low-risk studies undergo expedited review by a subset of the IRB.
- This process is quicker than full board review.
- Full Board Review (if applicable):
- Complex or higher-risk studies are reviewed by the entire IRB.
- Members discuss the proposal, raise questions, and vote on approval.
- Approval Decision:
- The IRB approves the study if it meets ethical standards.
- Researchers receive an approval letter.
- Ongoing Oversight:
- The IRB monitors the study throughout its duration.
- Researchers submit progress reports and seek re-approval for continuing research.
Remember that the IRB’s primary goal is to protect participants’ rights, safety, and well-being during research activities.